titanium valency|How to Find the Valence Electrons for Titanium (Ti) : Manila The valency of titanium is accurately four to attain stability. Valency is basically the integral properties of Titanium just like the other chemical element. The four valencies of Titanium states that it may .
Regino said members may pay their consolidated loan through a one-time payment within thirty (30) calendar days after receiving the approval notice or they may also opt to pay through installment. For the installment scheme, members must pay a down payment equivalent to at least 10% of the consolidated loan within thirty (30) calendar .
titanium valency,Valence : 1: Hydrogen (-1), +1: 2: Helium: 0: 3: Lithium +1: 4: Beryllium +2: 5: Boron-3, +3: 6: Carbon (+2), +4: 7: Nitrogen-3, -2, -1, (+1), +2, +3, +4, +5: 8: Oxygen-2: .
Properties. Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, .
Valency of Scandium: 21: 3: Valency of Titanium: 22: 4: Valency of Vanadium: 23: 5,4: Valency of Chromium: 24: 2: Valency of . The combining capacity, or valence, of O is apparently twice that of H or Cl. Two H atoms combine with one O atom in H 2 O So do two Cl atoms or two Li atoms (Cl .
116. 19K views 3 years ago. To find the number of valence electrons for Titanium (Ti) we need to look at its electron configuration. This is necessary because Ti .titanium valency How to Find the Valence Electrons for Titanium (Ti) The valency of titanium is accurately four to attain stability. Valency is basically the integral properties of Titanium just like the other chemical element. The four valencies of Titanium states that it may .
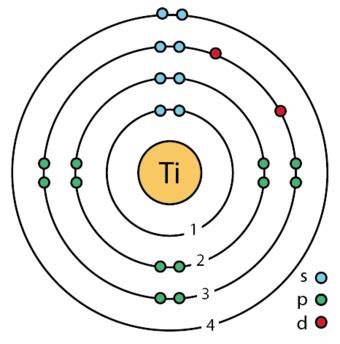
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, . Properties. Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4. Pure titanium is a lustrous white metal with low density, high strength, . Chemistry of Titanium is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Discovered independently by William .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called .titanium valencyTitanium is a chemical element of the periodic table with chemical symbol Ti and atomic number 22 with an atomic weight of 47.8671 u and is classed as a transition metal. . Valence electrons : 4: Valency electrons : 2,3,4: Bohr model: Electron shell for Titanium, created by Injosoft AB Ti. The valency of titanium is 3,4. The valency of Titanium is 2. This is because electronic configuration of it is 2,8,10,2. Valence describes how easily an atom or radical can combine with other chemical species. This is determined based on the number of electrons that would be added, lost, or shared if it reacts with . Titanium has four valence electrons. The nuclear number of titanium is 22 22 and has a place with the progress metal gathering. Note: We can write the total electron configuration for titanium is as per the following: 1s22s22p63s23p63d24s2 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 2 4 s 2. For progress metals, and specifically those in sections. Group 4 Elemental Properties. Because the elements of group 4 have a high affinity for oxygen, all three metals occur naturally as oxide ores that contain the metal in the +4 oxidation state resulting from losing all four ns 2 (n − 1)d 2 valence electrons. They are isolated by initial conversion to the tetrachlorides, as shown for Ti: The valency or valency chart is helpful in order to determine how many atoms of an element will combine with another element to form any chemical formula. Another important use of valency of elements is to find or deduce formulae of compounds. If we know the valency of elements, then we can easily write formulae of compounds of .

The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it’s oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one that .
The electron configuration for Titanium ion (Ti 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6. The number of valence electrons available for the Titanium atom is 4. Titanium is situated in the transition metal group and has an atomic number of 22. The orbital diagram for Titanium is drawn by following three principles – the Aufbau principle, Hund’s .
Chemistry of Titanium is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Discovered independently by William Gregor and Martin Klaproth in 1795, titanium (named for the mythological Greek Titans) was first isolated in 1910. Gregor, a Cornish vicar and amateur ..
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
How to Find the Valence Electrons for Titanium (Ti) Valence electron. The valence electron refers to the outermost electron in the outermost shell of an atom. Elements in the periodic table are arranged such that the elements in the same group will have the same valence electrons. For example, group 16 elements such as oxygen, sulfur, and selenium have 6 valence electrons.Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..Difference between Valency and Oxidation Number. Valency is different from the oxidation number, and it has NO SIGN. Thus, the valency of nitrogen is 3, whereas it can have oxidation numbers from -3 to +5. The .

Here, the electron configuration of this titanium ion (Ti 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2. This positive titanium ion (Ti 2+) has twenty-two protons, twenty-six neutrons, and twenty electrons. Also, titanium has two more ions. They are Ti 3+ and Ti 4+. Ti – 3e – → Ti 3+. The titanium(IV) ion, for example, is formed when the titanium atom loses its two 3d and two 4s electrons. These highest oxidation states are the most stable forms of scandium, titanium, and vanadium. However, it is not possible to continue to remove all of the valence electrons from metals as we continue through the series.Titanium dioxide, also known as titanium(IV) oxide or titania / t aɪ ˈ t eɪ n i ə /, is the inorganic compound with the chemical formula TiO 2.When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of .
The position of 3 elements A, B and C in the Periodic Table is shown above: Giving reasons, explain the following: (a) Element A is a non-metal. (b) Atom of element C has larger size than A. (c) Element B has a valency of 1.Titanium isopropoxide, also commonly referred to as titanium tetraisopropoxide or TTIP, is a chemical compound with the formula Ti{OCH(CH 3) 2} 4.This alkoxide of titanium(IV) is used in organic synthesis and materials science.It is a diamagnetic tetrahedral molecule. Titanium isopropoxide is a component of the Sharpless epoxidation, a method for the .
titanium valency|How to Find the Valence Electrons for Titanium (Ti)
PH0 · Valency Chart (Valency Table of Chemical Elements)
PH1 · Valences of the Elements Chemistry Table
PH2 · Titanium Valence Electrons
PH3 · Titanium Chemical & Physical Properties
PH4 · Titanium
PH5 · How to Find the Valence Electrons for Titanium (Ti)?
PH6 · How to Find the Valence Electrons for Titanium (Ti)
PH7 · Chemistry of Titanium
PH8 · 4.4: Valence
PH9 · 3.10: Valence Electrons